US chemists have found a way to regioselectively replace one of the carbon atoms in a benzene ring with a nitrogen atom, reports Science.
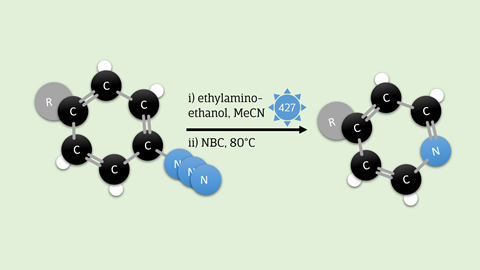
Medicinal chemistry uses molecules containing nitrogen atoms, such as pyridine. The search for new drugs therefore often involves a ‘nitrogen scan’, in which a nitrogen atom is placed at various points in the target molecule to see what effect it has on the drug target. Normally, this is a tedious process that involves redesigning the molecule multiple times to position the nitrogen atom elsewhere. Tyler Pearson, Mark Levin and colleagues at the University of Chicago have now found a simple solution. They developed a protocol that allows you to replace a carbon atom in a benzene ring with a nitrogen atom.
You attach an azide to your benzene ring and then shine blue light on it. This causes N2 to be released and the remaining nitrogen atom to be incorporated into the ring. You then react the resulting seven-membered ring with ethylamino-ethanol. If you then add a little base and slowly add N-bromocaprolactam, another six-membered ring - pyridine - is formed, with a carbene as a by-product, which is then oxidised.
The researchers tried about 20 variants, with overall yields ranging from 20 to 60%. They also compared a previously published synthesis of an azasteroid with their own protocol using a much cheaper building block. They increased the overall yield from less than 1% to 10%. In addition, the nitrogen scan was a success: using their method, it was indeed possible to make two pyridine variants in a straightforward manner.
‘Admitting that I am not entirely at home in medicinal chemistry, I can say that I find the overall transformation extraordinary’, says Jeroen Codée, professor of carbohydrate chemistry at Leiden University. ’You have to break the stable aromaticity of the benzene ring and push out the right carbon atom. The fact that you can do a nitrogen scan like this at a later stage is also very useful, so you don’t have to rebuild your molecule from scratch. I can see this being used in future medicinal projects.’
Pearson, T.J. et al. (2023) Science 381, DOI: 10.1126/science.adj5331












Nog geen opmerkingen