One of the best-known chemical reaction mechanisms – the SN2 substitution – sometimes proceeds in a completely different way than expected, as quantum chemical models by a team from Leiden and Amsterdam show.
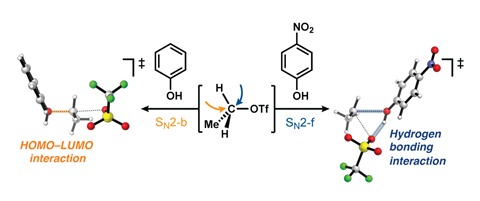
It’s one of the first chemical reaction mechanisms you learn about: the bimolecular nucleophilic substitution (SN2) reaction, in which the attacking particle (nucleophile) attacks the substrate (electrophile) from behind and the leaving group leaves the substrate at almost the same time (see image below). However, Wouter Remmerswaal, Jeroen Codée, Thomas Hansen and colleagues observed reaction products that could not be explained by this mechanism in their experiments eight years ago. Now they have finally solved part of the puzzle, as they show in Chemistry - A European Journal.
‘In 2016, Wouter did his master’s internship in Jeroen’s lab at Leiden University, where I was doing my PhD at the time’, explains Thomas Hansen, associate professor at VU University Amsterdam. ‘We were working on complex substitution reactions and came up with results that we still cannot fully explain.’
The project came to a halt until the researchers picked it up again about three years ago. ‘We simplified the reactions and put them into the computer’, continues Hansen. ‘When we looked at these substitution reactions again, it turned out that the traditional SN2 reaction is not always the most favourable mechanism; sometimes the nucleophile comes from the front rather than the back. That’s something that we hadn’t considered in 2016, but it might complete the picture.’
Backside vs. frontside
Specifically, they looked at quantum chemical models of nucleophilic substitutions between phenols and alkyl triflates, and how the ‘normal’ backside variant (SN2-b) compares to the less common frontside variant (SN2-f). Professor of Organic Synthesis Jeroen Codée says: ‘We teach our students that SN2 always comes from the backside, so the possibility that it could come from the other side was less obvious. But in these model reactions we see that it is quite logical that the nucleophile can come from the front, because the alcohol group can give its proton directly to the leaving group.
The frontside SN2 does not come out of the blue. Remmerswaal explains that this mechanism is often found in enzymes, and in the (older) literature you can also see that more conversions are affected by it. In SN2-b there is an inversion of the stereochemistry, whereas in SN2-f there is stereochemistry retention. In solvolysis reactions, where the solvent does an SN2 reaction, you see a surprisingly high retention rate, which seems to indicate the SN2-f mechanism.
Trends
The researchers found two clear trends, one related to the degree of substitution of the electrophile and the other to the strength and acidity of the alcohol nucleophile. ‘An electrophile with more substituents and a weaker nucleophile sends to the front side’, Hansen explains. ’If the electrophile has more groups around it, more carbocationic character can be built up during the substitution reaction. This allows the leaving group to make room for the nucleophile.’
The other trend concerns the nucleophile: the weaker it is, the more acidic its character is. In other words, a weak electrophile can give up its proton more easily, which in turn is of interest to the leaving group, as this proton stabilises the leaving group and causes it to leave more quickly. The hydrogen bridge interaction between the alcohol and the leaving group thus plays an important role at the frontside mechanism.
But quantum chemistry is not the end of the story, as the chemists are also putting these models into practice. ‘We are now looking at a number of reactions where the frontside seems to be a realistic scenario’, says Codée. ‘But before we go public with this, we want to have a firmer experimental basis. A lot is still unclear, but eventually you want to be able to map the whole interaction between standard SN2, SN1 and SN2 frontside and thus make predictions about what happens in substitution reactions.’
Textbook substitutions
Remmerswaal: ‘The tricky thing about this kind of research is that once you have a piece of the puzzle and an understanding of where it should be, then other pieces of the puzzle come out from under the carpet. These seem to be added in secret, which doesn’t make the process any easier.’
All in all, ‘textbook substitutions are not necessarily what they seem’, says Codée. ‘It’s another example that we are still a long way off.’ Hansen concludes: ’Although SN2 is the simplest organic reaction, it is also the most complex.’
Remmerswaal, W. et al. (2024) Chem. Eur. J. e202400590, DOI: 10.1002/chem.202400590












Nog geen opmerkingen