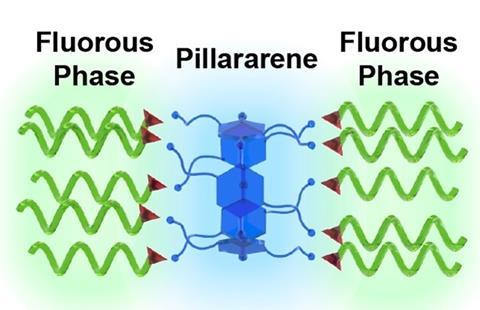
A new method for filtering PFOS and PFOA out of water has been developed by an international team, as reported in Angewandte Chemie International Edition. The method uses ring-shaped molecules with a functionalised rim, which allows for very efficient and selective filtration.
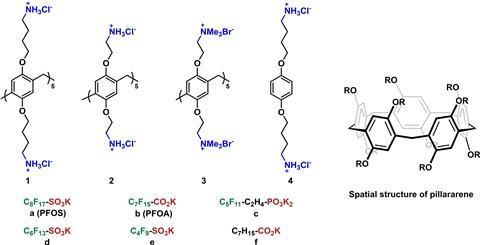
The problem of PFAS contamination is well-known, but the focus is now shifting to finding solutions. Take water purification, where PFAS (and pretty much everything that floats through water) is partly captured by, for example, activated carbon. ‘Activated carbon is neither selective nor very efficient’, says Han Zuilhof, professor of organic chemistry at Wageningen University & Research and Tianjin University in China. ‘You can extract PFAS with it, but the capacity is low and it is effectively one-time use.’ Zuilhof and his team have now created molecules that do a much better job: deca-ammonium-functionalised pillar[5]arenes.
Exercise
However, this has taken almost a decade of research, he explains. ‘In Tianjin, we have been working for about nine years on those so-called pillararenes, ring-shaped molecules consisting of benzene rings which you can functionalise on both sides with groups of your choice.’ A few years back, they made a macrocycle with minimal distinction between both sides of the ring: on one side, CH3 groups and on the other, very selectively, only CD3 groups. ‘It’s of no use at all, but it was a wonderful exercise and shows what can be done.’
They kept up that curiosity-driven approach in the Netherlands. ‘With colleague Harry Bitter, we looked at the possibility of fixing the pillararenes to a surface and putting something inside the ring,’ says Zuilhof. ‘The idea was to put a catalyst in it that you can take out when it’s “finished”.’ But the research took an unexpected turn when one of the PhDs found out that for certain compounds, the ratio was not one to one. ‘What we found was that if you apply a positive charge at all attachment points in the form of chains with a final ammonium, acids can attach to them through electrostatic bonds as well.’
This already works to some extent with alkanoic acids, which bind well electrostatically but have relatively little van der Waals interaction with each other. It works much better with perfluoroalkanoic acids. Zuilhof: ‘The first perfluoroalkanoic acid attaches and detaches quite easily, but as soon as more of them start to bind to our molecule, the fluorine chains start interacting very strongly with each other and stick tightly to the pillarene.’ It basically boils down to a two-component binding mechanism.
PFOA and PFOS
A molecule to which PFAS adhere offers obvious opportunities. In the paper, Zuilhof and colleagues showed how they attached the pillararenes to TentaGel S, a commercial resin, so that it could act as a kind of filter for perfluorooctanoic acid (PFOA) and perfluorooctanesulphonic acid (PFOS). This worked well in the lab: they filtered heavily contaminated water (10 mg PFAS per litre) to a PFAS concentration of less than 50 ng/L within minutes. Activated carbon only achieved 230-515 µg/L in a similar experiment – ten thousand times worse.
Of course, the lab is not the same as the real world, where there is little competition from other substances found in the river. ‘But our molecules also work well with real river water’, says Zuilhof. ‘It often contains humic acids in concentrations thousands of times higher than PFAS, which also compete with them. So you can see that these humic acids first bind to the pillararenes and the PFAS slowly but surely replace them. That shows that our molecules work very specifically, and that’s really a step beyond what’s been possible before.’
Drinking water
What are Zuilhof’s concrete ideas? ‘The first thing I would like to target is industrial wastewater, because you are dealing with relatively high concentrations. So tackle the problem at the source’, says Zuilhof. ‘Secondly, drinking water companies are of course a logical target. Drinking water really has to be clean, and our pillararenes can help with that in the right way.’
To do this, you have to attach them to something hard that the water can flow over. ‘It is still a challenge, especially scaling up, which needs a lot of chemical and technological work. You need the whole chain to work together: universities, knowledge institutes and companies.’ For the latter group, water purification is a tricky issue, says Zuilhof. ‘They would like to improve their waste flows, but they don’t dare say so, because then they would have to admit that there is a problem. PR is tricky. That’s why I’m trying to make a case for them, so that they’re not just frowned upon. The story has to be: “The pollutant is used because ultimately the customer wants products with certain properties. So the industry is working hard on alternatives, but in the meantime we want to get rid of it cleanly. Such a perspective hopefully provides starting points for cooperation and improvement. I hope that companies will come to us, we are happy to work with them on this.’
Essential expertise
Another practical point to consider is the number of types of PFAS. The international team has demonstrated the effect of pillararenes on two of the hundreds of species found in water. ‘That’s where the years of playing with these pillararenes come in handy, as we now know what we can change because we’ve tried so many things’, says Zuilhof with a laugh. ‘But that requires some more research. You could even modify the ring to trap other substances, such as parabromo compounds. But then you’re talking about something completely different.’
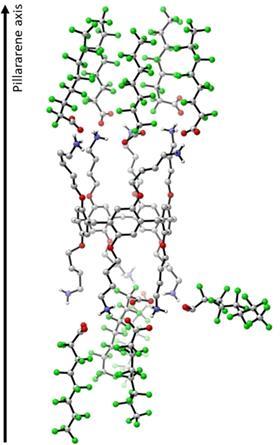
Zuilhof is also very pleased with the team. ‘The Russian Fedor Miloserdov, with whom I coordinated this project, a Brit, a German, Chinese in the Netherlands and China, but also, for example, a student from Wageningen. Each brought a specific, essential expertise to the table’, he explains. ‘For example, we calculated quantum chemical fluorine-fluorine interactions, one of the most difficult interactions to model. But we also needed expertise in the synthetic part, or surface chemistry. And then there is the analytical side. We wanted to show the difference between three and four nanograms per litre! Determining the stoichiometry was also a challenge, for which we determined the crystal structure with a new X-ray from Bruker that they had to work on a lot. In short, every single author was needed.’
Gao, T-N. et al. (2024) Angew. Chem. Int. Ed. e202403474, DOI: 10.1002/anie.202403474












Nog geen opmerkingen