Using specialised NMR methods, you can discover what the reactive intermediates are in reactions of sugar molecules, says a paper in JACS.
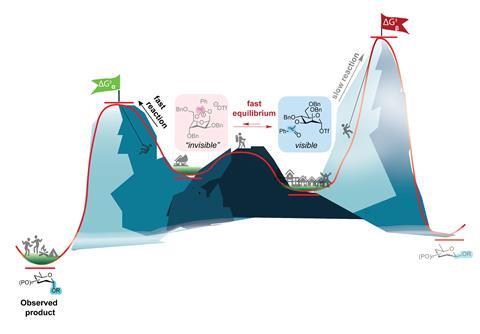
If you work with sugar molecules, you almost automatically run into the problem of ‘stereoselective glycosylation reactions’: how do you get one sugar molecule to another sugar molecule in the intended way? ‘That glycosylation reaction mechanism can be very simple’, says Thomas Boltje, associate professor of chemical biology at Radboud University (RU). ‘The reactive intermediate is in some cases easy to detect with, for example, NMR, and explains the formation of the reaction product. But in many cases you see a reaction intermediate that cannot possibly lead directly to the product you made. In these cases, the structure of the “real” reactive intermediate is unknown.’
Hypothesis
A hypothesis for this problem has existed for years that these reactions proceed via other reactive intermediates that are in equilibrium with the detected intermediate, but providing evidence for this is difficult. ‘The structures that form are unstable, so they revert to more stable forms and does not make detection easy’, Boltje continues. In ‘a real team-effort’, Boltje, Frank de Kleijne, Floor ter Braak, Paul White and colleagues have now used a method that allows you to understand much better what exactly is happening; this, in turn, provides tools to do more targeted glycosylations.
‘We can directly detect the “invisible” intermediates without observing them directly’
Paul White
When pairing two sugar molecules – glycosylation – you often install a good leaving group on the first carbon atom next to the oxygen atom in your ring. Because glycosylation falls into a spectrum of SN1 and SN2 reactions, you have several stages that the molecule goes through (before reacting), all of which are in equilibrium with each other: from a covalent bond between sugar and triflate to being completely separate from each other, resulting in a triflaation and a glycosyl ion.
Specialised NMR
‘We used some more specialised NMR methods to capture the kinetic process’, says junior research scientist Frank de Kleijne. ‘Those are exchange spectroscopy NMR [EXSY, ed.], or chemical exchange saturation transfer NMR [CEST, ed.] which is already widely used in proteins or in MRI. This allowed us to use the chemical equilibrium between the clearly visible intermediate and the highly reactive intermediate as a reporter.’
Paul White, facilities manager and the group’s ‘NMR guru’, adds: ‘With EXSY you look at two distinct and highly visible populations: you excite one population and see how it transfers to the other. You can then measure the kinetics of the reaction and make models. In the other method, CEST NMR, one population is visible and the other is not, or barely’, he explains. Both methods help to reveal the intermediates. ‘We can directly detect the “invisible” intermediates without directly observing them’, says White.
‘The easily detectable reaction intermediate is not necessarily the reactive intermediate that forms the product’
Thomas Boltje
Specifically, they looked at eight commonly used glycosyl donors (glucose and mannose variants) that Floor ter Braak had synthesised. In previous research, she had studied these molecules in the gas phase using infrared ion spectroscopy (IRIS). ‘In the gas phase you can define extremes, but the disadvantage is that the behaviour of molecules in the gas phase is not necessarily representative of molecules in solution.’
‘That is why we went back to NMR measurements’, adds Boltje. ‘The eight donors have already been studied a lot. With these methods, we were able to create a dynamic picture and thus determine reaction rates, so you could see how the reactions proceeded. The theory proved correct in most cases, although there was an exception for a mannosyl triflate. There is a lot of debate in the field about whether or not neighbouring group effects are involved, and we showed that this is indeed the case and that it affects glycosylation.’ So, the easily detectable reaction intermediate is not necessarily the reactive intermediate that forms the product.
View window
‘What was surprising in the research was that the well-observable alpha-triflate signal became another well-observable NMR signal: that of the triflate anion’, says De Kleijne. ‘This was so surprising that it really kick-started our research.’ Of course, when the triflate is released, something from the sugar group must remain.‘But what was it? And how do you measure it? It caused a lot of discussion in the group’, says Boltje. ‘We had to hunt and tinker to find out what happened to the sugar part, but one night we got a message from Frank: “We’ve found the intermediate!”.’
‘We learned that there is a ‘window’ in which you can follow this kind of reaction process’, says Boltje. ‘There are all sorts of parameters that affect whether you can see a particular intermediate or not.’
Practical laboratory
The beauty of this research is its broad applicability and accessibility. After all, it’s not just glycosylation reactions that can be studied with this method, as White explains: ‘Anything with a slow equilibrium intermediate in the reaction can theoretically be studied using this method. And almost every university has NMR equipment, so you can use it anywhere in the world.’ De Kleijne, who has written a very comprehensive tutorial on the NMR methods in the supporting information, adds: ‘Most of our measurements were done on a 300 MHz NMR from the practical lab, that’s how accessible it is.’
The next step? Among other things, there’s a plan for a database, says De Kleijne: ‘We’re now working on mapping what happens when you change something about your sugar, based on basic experimental data that tell you what the reaction conditions should be.’
‘There are many more reaction intermediates that we would like to detect with this method. Ultimately, we would like to be able to measure and understand complete subsequent reactions’, says Boltje. ‘It should become less of a trick and more of real science.’
De Kleijne, F.F.J. et al. (2023) JACS, DOI: 10.1021/jacs.3c08709












Nog geen opmerkingen